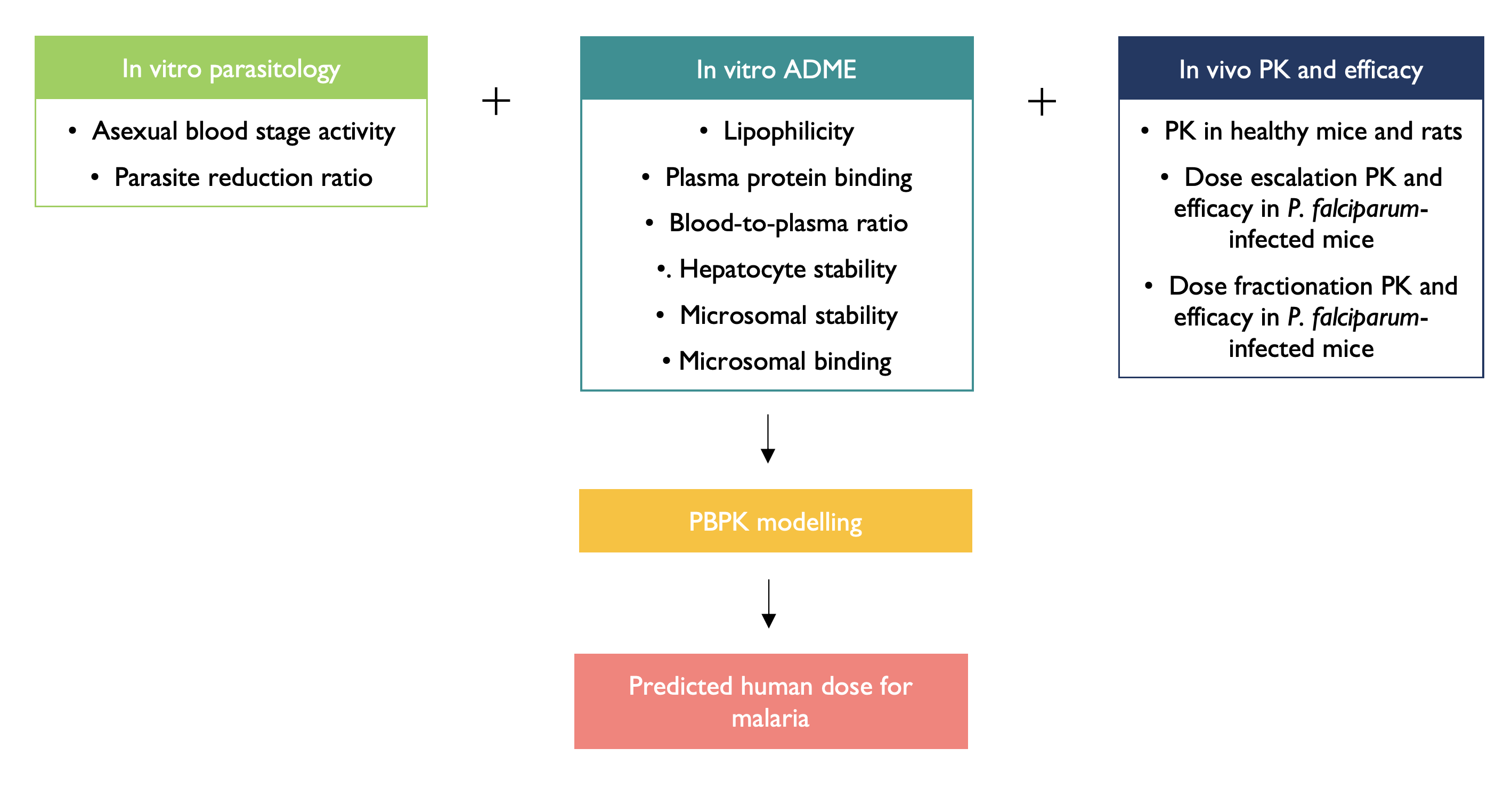
We are investigating whether clinical anticancer kinase inhibitors can be repurposed for the treatment of malaria. Physiologically based pharmacokinetic (PBPK) modelling of in vitro and in vivo data is used to perform human dose predictions, with the overall objective of comparing how the kinase inhibitor’s predicted human dose for malaria differs to the maximum tolerated dose that is used clinically for cancer.
A multiparameter optimisation of the predicted human dose will be achieved using PBPK models which incorporate antiplasmodium activity with the drug-specific physicochemical properties that govern the in vivo drug disposition. The pharmacokinetic/pharmacodynamic (PK/PD) relationships will be evaluated in a Plasmodium falciparum-infected humanised murine model, which will provide a better prediction of efficacy against the clinically relevant species of malaria, compared to the standard murine models which are infected with host-specific rodent forms of Plasmodium. With a focus on safety, the predicted human doses will inform the potential to repurpose anticancer kinase inhibitors for malaria.